We're Sending Enzymes Into Space
USA Health medical research aboard NASA launch.
A protein developed by researchers at the University of South Alabama College of Medicine will launch into space on April 18, 2014, as part of the SpaceX Commercial Resupply Services (CRS) mission to the International Space Station. The enzyme, a protein phosphatase called PP5, will launch on the Falcon 9 rocket and is contained in the DragonLab, the cargo craft attached to the top of the rocket.
The protein will be placed in incubators on the International Space Station, and after a period of approximately 3.5 months it will return to Earth. The protein is one of 92 proteins – produced by several laboratories in the United States, England and Germany – that are being delivered to the station.
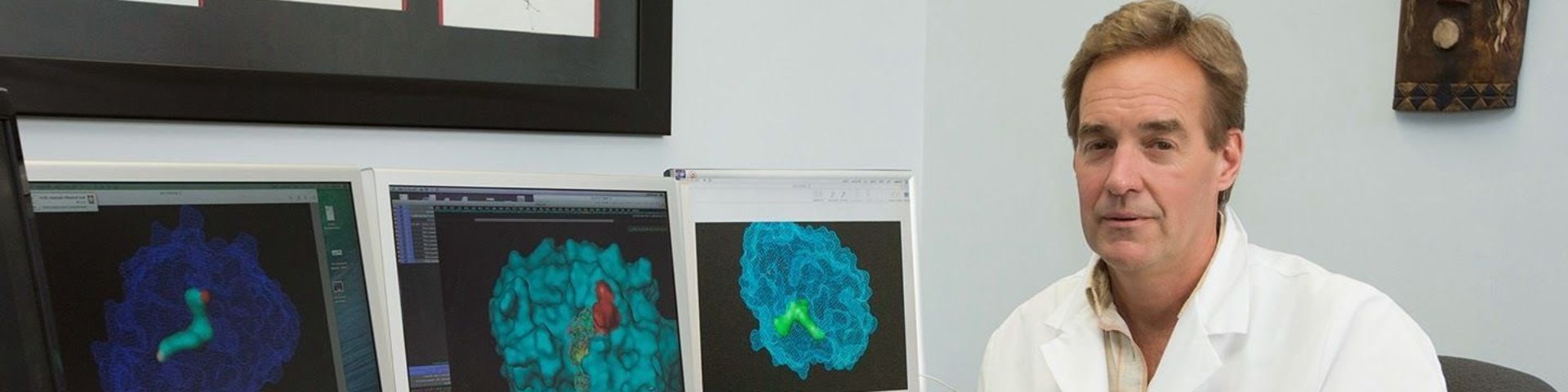
According to Dr. Richard E. Honkanen, professor of biochemistry at the USA College of Medicine and lead researcher on the PP5 project, PP5 is considered a validated target for anti-tumor drug development. “Our studies in the past 15 years have revealed that PP5 is over-expressed in human breast cancer,” he said. “In mouse models of tumor development, the over-expression of PP5 aids tumor growth.”
At the cellular level, Dr. Honkanen said PP5 appears to provide a survival advantage to cancer cells in low oxygen. “This may help the cancer cells stay alive in the early stages of metastatic disease,” he said. “Therefore, an inhibitor of PP5 may kill metastatic cancer cells before they start growing into new tumors.”
However, Dr. Honkanen said the development of PP5-specific inhibitors has proven very difficult. “To aid the development of PP5-specific inhibitors we desire detailed information about the active site, the part of the enzyme where catalysis occurs.”
PP5 acts to catalyze the removal of phosphate from proteins that control cell growth and survival. The removal of phosphate turns on processes that allow cancer cell growth and proliferation. “If we inhibit this key step,” he said, “we hope to prevent cancer cell growth/survival.”
The International Space Station, which serves as a microgravity research laboratory, will use the proteins to grow large crystals of PP5. This will allow Dr. Honkanen’s lab to utilize an emerging technology called neutron crystallography, a very powerful technique for protein structure determination. “For neutron crystallography, we need very big crystals, and to date this has not been done,” he said. “In theory, we will be able to grow bigger crystals in space than we can on Earth.”
Dr. Mark Swingle, a research associate in Dr. Honkanen’s lab, previously worked with Dr. Eva Ciszak at NASA to develop methods to make crystals of PP5. Their pioneering studies rebuilt PP5 at the molecular level using complex computer programs. Dr. Honkanen hopes neutron crystallography will provide an even higher (atomic level) resolution.
“Positions of hydrogen atoms in a protein structure can be directly determined with much greater precision and confidence by neutron crystallography,” Dr. Honkanen said. “The ultra high-resolution structure will help us design and synthesize specific inhibitors.”




